PA14 Oropharyngeal Colonization Model in CF Mice
Dependence of chronic oropharynx colonization on:
- CFTR expression
- PA14 virulence factors
| Click thumbnail at right for larger image. | |
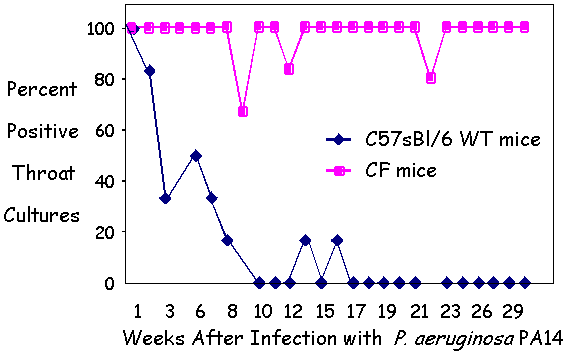 |
| Figure 1 - Chronic PA14 infection of the
oropharynx of CF Mice. |
I. PA14 chronically colonizes the oropharynx of CF Mice
The laboratory of Dr. Gerald Pier, collaborating with us as a part of the ParaBioSys PGA, established a protocol to chronically infect transgenic cftr knock-out mice (CF mice) with P. aeruginosa PA14 under conditions in which wild-type mice did not become chronically infected (Coleman, F.T., et al Proc Natl Acad Sci U S A 100:1949-1954).
| Click thumbnail at right for larger image. | |
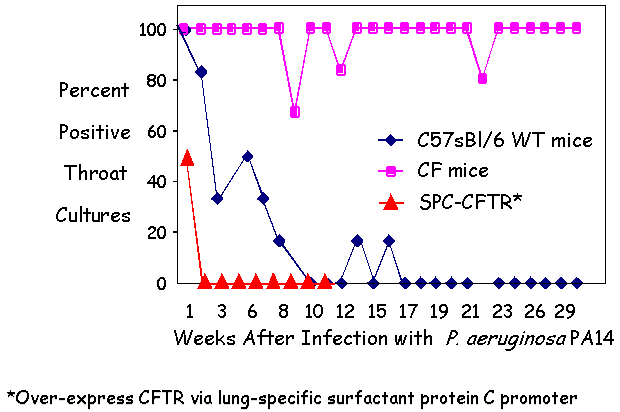 |
| Figure 2 - CFTR overexpression confers enhanced
clearance of PA14 in CF Mice. |
II. Dependence of chronic oropharynx colonization on: CFTR expression
The Pier lab further engineered CF mice to over-express human CFTR in the lung under the control of the surfactant protein C promoter (SPC-CFTR mice). The SPC-CFTR mice exhibited accelerated clearance of P. aeruginosa from their respiratory tract compared with wild type mice.
| Click thumbnail at right for larger image. | |
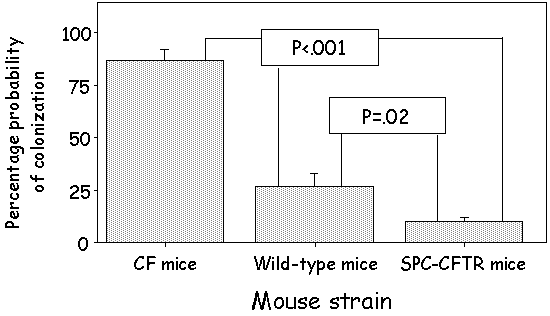 |
| Figure 3 - Summary of Results from Seven
CF Mouse Colonization Studies. |
Overall, the results of several CF mouse colonization studies revealed a significantly higher likelihood of obtaining a positive throat culture in CF mice compared to wild-type mice, as well as a significantly lower likelihood of obtaining a positive P. aeruginosa culture from the SPC-CFTR mice compared with wild-type mice.
| Click thumbnail at right for larger image. | |
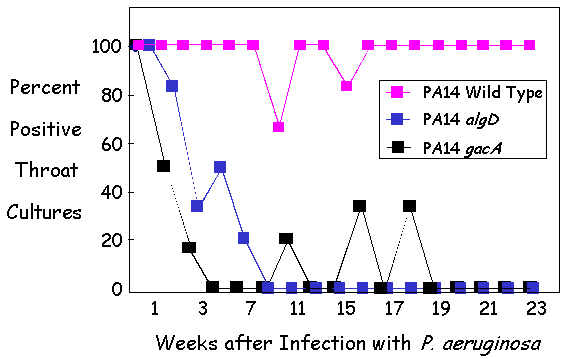 |
| Figure 4 - PA14 virulence factors are essential
for oropharyngeal colonization of CF Mice. |
II. Dependence of chronic oropharynx colonization on: PA14 Virulence Factors
The Pier lab has also demonstrated that two known P. aeruginosa
virulence factors were shown to be required to establish and maintain
colonization. Both the GacA global regulator of gene transcription and the
alginate surface polysaccharide of the bacterium were required for
colonization by P. aeruginosa strain PA14. Complemented strains either
fully or partially reacquired the parental phenotype (not shown). Thus a
relevant murine model of chronic P. aeruginosa infection in the setting
of a CF phenotype was successfully developed, which will be critical for
further definition of P. aeruginosa virulence factors relevant to
pathogenesis (Coleman et al, 2003 Proc. Natl. Acad. Sci. USA3). PA14 mutants
from the non-redundant
transposon insertion library shown to be required for pathogenesis in
other model hosts, will be tested in this CF oropharyngeal colonization model.
In this way, we hope to identify P. aeruginosa virulence factors
involved in the pathogenesis of CF.
 Back to Host-Pathogen Interactions Back to Host-Pathogen Interactions
| 



